SYSTEMATICS OF THE GENUS CAREX
by Anthony Darrouzet-Nardi · 5 May 2003
Introduction
 edge taxonomy began in 1753 when Carl Linnæus assigned twenty-nine sedge species to the genus Carex in his book Species Plantarum (Linnæus 1753 pages 972-979). Since Linnæus, botanists have divided the sedges into 2,000 species in more than 70 sections in varying numbers of tribes or subgenera. Carex resides in the family Cyperaceae with other similar graminoid monocots. Sedge species are morphologically homogenous; Carex and its closely related genera are not distinguishable by vegetative characters alone (Metcalfe 1969). This makes sedge taxonomy challenging.
edge taxonomy began in 1753 when Carl Linnæus assigned twenty-nine sedge species to the genus Carex in his book Species Plantarum (Linnæus 1753 pages 972-979). Since Linnæus, botanists have divided the sedges into 2,000 species in more than 70 sections in varying numbers of tribes or subgenera. Carex resides in the family Cyperaceae with other similar graminoid monocots. Sedge species are morphologically homogenous; Carex and its closely related genera are not distinguishable by vegetative characters alone (Metcalfe 1969). This makes sedge taxonomy challenging.
In 1990, shortly before molecular systematics revolutionized biological taxonomy, Anton A. Reznicek wrote an authoritative review entitled "Evolution in Sedges (Carex, Cyperaceae)." (Reznicek 1990). He summarized the many - and often unsuccessful - attempts to classify sedges. He then distilled the most plausible evolutionary hypotheses explaining relationships between sedge species. In 1995, Jeremy J. Bruhl used cladistic and phenetic analyses of morphological data to propose hypotheses describing the place of Carex and its related genera within the family Cyperaceae (Bruhl 1995). As Reznicek (1990) points out, significant evolutionary paradoxes remain even with the most careful analyses of morphological data.
The morphological homogeneity of Carex makes the genus a prime candidate on which to use molecular methods to resolve evolutionary history. Since 1990, a number of studies have used molecular data to test Reznicek's and Bruhl's hypotheses. I will explain the hypotheses that they put forth about Carex evolution and discuss how the hypotheses have held up in the light of molecular phylogenetic evidence.
Pre-molecular hypotheses of Carex evolution
Kükenthal (1909) divided Carex into 4 subgenera: Primocarex, Vignea, Indocarex and Eucarex (usually called just Carex). Kenneth K. MacKenzie (1931, 1935) wrote the first comprehensive analysis of sedge diversity. He separated Carex into 71 sections. He placed Carex and the genera Kobresia, Uncinia, and Cymophyllus in the tribe Cariceae, one of five tribes that he recognized within the family Cyperaceae. Table 1 summarizes this system. Although Reznicek (1990) cites a number of other studies that examine Carex, these two works laid an enduring foundation for Carex systematics. Morphological analyses of Carex have focused on inflorescence, rachilla, and perigynium morphology. (The perigynium is a sac-like structure that subtends the gynoecium.) Reznicek (1990) analyzes these features in detail to update these foundational works.

The subgenus Primocarex was defined by the presence of only one terminal spikelet in the inflorescence (unispicate), instead of also having multiple lateral spikelets. Reznicek cites multiple lines of morphological evidence suggesting that Primocarex is polyphyletic and that unispicate Carex species have evolved many times. Carex researchers have suggested that Carex may be derived from a Kobresia- or Schoenoxiphium-like ancestors. Reznicek called this suggestion into doubt citing patterns of inflorescence diversity. In the case of Kobresia, he said that there may not be a strong phylogenetic distinction between it and Carex. Subgenus Indocarex shows the most primitive features. Reznicek suggested that the other two subgenera, Carex and Vignea, may have evolved from Indocarex-like ancestors. However, he also noted that there is not enough evidence to say this definitively. He could not eliminate the possibility that Indocarex is a separate evolutionary clade which is more closely related to other genera in the Cariceae tribe.
Reznicek (1990) primarily focused on evolution within the genus Carex. Bruhl (1995) proposed a new system of classification for the 122 genera he recognized within the family Cyperaceae. Carex, by far the largest genus, accounts for 2,000 of the 5,000 species in the Cyperaceae. Bruhl discarded MacKenzie's five tribes. Instead, he recognized two sub-families: the Cyperoideae, consisting of four tribes, and the Caricoideae, with six tribes including Cariceae. He admitted that only three or four of the ten tribes in his system are likely monophyletic. He hypothesized that Cariceae is monophyletic. Goetghebeur (1998) proposed an alternative system to that of Bruhl (1995). His system was originally published in Dutch, so I cannot evaluate his hypotheses. It included four subfamilies and fifteen tribes. Table 2 shows a summary of Reznicek's and Bruhl's hypotheses.
Molecular insights on Carex evolution
Yen and Olmestead (2000) used chloroplast DNA to analyze relationships within the tribe Cariceae. They recognized five genera in the Cariceae: Carex, Kobresia, Uncinia, Cymophyllus, and Schoenoxiphia. They analyzed 29 taxa in the Cariceae and four outgroups. They included Carex species from each of Kükenthal's four subgenera: Primocarex, Vignea, Carex, and Indocarex.
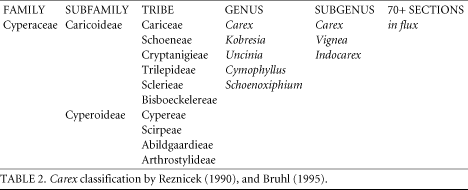
Their data had direct bearing on several of Reznicek's (1990) hypotheses. Subgenus Primocarex came out as a clearly polyphyletic clade as suspected. Subgenus Vignea came out as a well-supported group. Kobresia's place within the Caricieae was uncertain in Yen and Olmstead's analysis, but it was clear that Kobresia is not a separate group from Carex. The generic boundaries are blurred. Their data suggested two possible places for Kobresia species: either at the base of the Carex clade, or nested well within the clade. Their data also suggested that Uncinia is derived from within Carex. Uncinia has distinctive hook-shaped rachillae that most researchers thought to be a primitive feature. Subgenus Carex, as it is circumscribed today, appears to be a polyphyletic genus, but more work must be done to determine the exact nature of the relationships.
Yen and Olmstead supported Bruhl's (1995) hypothesis that Cariceae is a monophyletic group. Their analysis placed Schoenoxiphium sister to the rest of the tribe, suggesting that Carex was derived from a Schoenoxiphium-like ancestor. Schoenoxiphium is a small genus of 17 species found in southern and eastern Africa and Madagascar (Kukkonen 1986).
Roalson et al. (2001) used nrDNA and cpDNA to analyze 100 Carex species, and 16 other species within the Cariceae. They looked most closely at the relationships between the genera within the Cariceae. They found similar but not identical results. They found Kobresia and Uncinia to be nested within Carex. Kobresia was nested well within Carex, not at the base of the lineage, as Yen and Olmstead thought was possible. Once again, Vignea came out as a well-supported group. Instead of finding Schoenoxiphium to be sister to the rest of the tribe, Roalson et al. suggested that it too was derived from within Carex. They propose three major clades within the Cariceae: a basal clade of the subgenus Vignea, a clade with mostly Carex species from subgenera Indocarex and Carex, and a clade with many of the unispicate Carex species (Primocarex) and the other four genera in the Cariceae (Figure 1). More comprehensive molecular work may help to clear up remaining discrepancies. Each of these studies used only a small fraction of the 2,100 species within the Cariceae.
Many studies have addressed systematic questions within Carex at the sectional level (e.g., Miyamura et al. 1995, Waterway et al. 1997, Starr et al. 1999). Discussion of the detail in these studies is beyond the scope of this paper. Clearly, many of the original sections defined by MacKenzie (1931, 1935) would not hold up to tests of monophyly. Egorova (1999) and Reznicek (2001), have improved the sectional circumscriptions, but it appears that detailed molecular work will have to be done in each area of Carex to determine its complete evolutionary history.
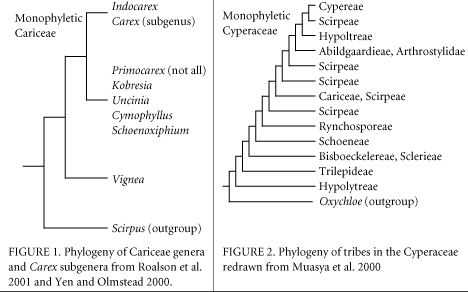
Plunkett (1995) and Muasya et al. (1998, 2000) address the question of the position of the Cariceae and other tribes within the Cypearaceae. All of these studies used rbcL sequence data to determine relationships. Muasya (2000) combined the rbcL data with an extensive morphological data set from Bruhl (1995) and is the most comprehensive analysis. All of these studies found that sub-family and tribe classifications in neither Goetghebeur (1998) nor Bruhl (1995) contained many well-supported groups (Figure 2). The Cyperaceae as a whole were found to be monophyletic in Muasya et al. (1998) and Muasya et al. (2000) but not in Plunkett (1995). The combined morphological/molecular data set and larger number of taxa that were evaluated in Muasya et al. (2000) make the most convincing case. It seems probable that the Cyperaceae are monophyletic. None of these studies provided evidence that the Cariceae were not monophyletic, which supports the conclusions of the studies that focused on the Cariceae genera.
Conclusion
The hypotheses set out by Reznicek (1990) to explain evolutionary trends within Carex have held up fairly well, considering the convolutions of sedge systematics. Some of the more clear hypotheses such as the polyphyly of the subgenus Primocarex were strongly supported by the molecular studies. Reznicek's suspicion that the boundary between Carex and Kobresia was artificial, despite many studies suggesting that Carex evolved from a Kobresia-like ancestor was remarkably prescient. There were also unexpected results from the molecular studies such as the apparent derivation of Uncinia from within Carex. Of course, there have only been a handful of rigorous molecular analyses of these taxa. Any support or lack of support for the hypotheses that Reznicek and others proposed is tentative.
The division of larger groups within the Cyperaceae is a more complex problem. Before molecular systematics, there was much less agreement on reasonable ways in which to classify the genera within the family. Systems proposed by Kükenthal (1909), Goetghebeur (1998), and Bruhl (1995) were widely divergent. Each author acknowledged the inadequacies of their systems. While studies like Muasya's (2000) have helped to clear up certain points such as the monophyly of the tribe Cariceae, the Cyperaceae clade is still a daunting mystery.
At each recognized taxonomic rank within the Cyperaceae - family, subfamily, tribe, genus, subgenus, section, and species - researchers have identified some monophyletic groups and been forced to lump the remaining taxa into paraphyletic or polyphyletic groups. The problems with the traditionally ranked botanical taxonomic system are particularly acute in Carex and the rest of the Cyperaceae. The genus Carex creates an enormous taxonomic wealth gap: it has 40% of the species in a family with 122 genera. There are only three ranks used within the genus: subgenus, section and species. These ranks are not sufficient to describe all of the monophyletic groups that have been and will be identified in a properly hierarchical system. Within the Cyperaceae as a whole, the subfamily and tribal classifications are largely inadequate, even with molecular data that is available to date. Thus, the few good groups, such as Cariceae, risk becoming the baby that is thrown out with the bathwater - in this case the poorly circumscribed tribes that researchers such as Bruhl and Goetghebur are compelled to invent. Researchers looking to test a rankless taxonomic system should consider using Carex and the Cyperaceae.
Reznicek (1990) points out that while molecular studies may cut through the confusing and homologous morphologies in Carex, they will not necessarily explain the evolutionary progression which created the observed modern morphologies. The results of the studies that have been done thus far validate this concern. Molecular data has provided needed insights on the interrelationships between the taxa, but so far have not been able to provide taxonomic resolution fine enough to allow us to infer evolution of particular morphologies such as rachilla structure or perigynium shape. Molecular studies have made important strides in developing subgeneric and to some extent sectional classifications within Carex. They have also added to our understanding of how Carex fits with the rest of the Cyperaceae. A combination of both traditional morphological studies and more molecular studies will help answer the formidable questions that remain.
Literaure Cited
Bruhl, J. J. 1995. Sedge Genera of the World: Relationships and a New Classification of the Cyperaceae. Australian Systematic Botany 8:125-305.
Egorova, T. V. 1999. The Sedges (Carex L.) of Russia and Adjacent States (within the limits of the former USSR). St. Petersburg State Chemical-Pharmaceutical Academy, St. Petersburg and Missouri Botanical Garden, St. Louis.
Goetghebeur, P. 1998. Cyperaceae. Pages 141-190 in K. Kubitzki, editor. Flowering plants. Monocotyledons: Alismatanae and Commelinanae (except Graminae). Springer-Verlag, Berlin ; New York.
Kükenthal, G. 1909. Cyperaceae - Caricoideae. Pages 1-824 in A. Engler, editor. Das Pflanzenreich. Wilhelm Englemann, Leipzig.
Kukkonen, I. 1986. Schoenoxiphium. Pages 345-405 in O. M. Hilliard and B. T. Burtt, editors. Notes on some plants of southern Africa chiefly from Natal: XIII. Notes Royal Botanical Garden, Edinburgh.
Linnæus, C. 1753. Caroli Linnaei Species plantarum, exhibentes plantas rite cognitas, ad general relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum system a sexuale digestas. Impensis L. Salvii, Holmiæ,.
MacKenzie, K. K. 1931. (Poales) Cyperaceae - Cariceae. North American Flora 18:1-168.
MacKenzie, K. K. 1935. (Poales) Cyperaceae - Cariceae. North American Flora 18:169-478.
Metcalfe, C. R. 1969. Anatomy as an Aid to Classifying the Cyperaceae-M. American Journal of Botany 56:782-790.
Miyamura, Y., T. Okigaki, and T. Hoshino. 1995. Phylogenetic studies of Carex section Praecoces (Cyperaceae) by restriction fragment analysis of chloroplast DNA. Bulletin of the Okayama University of Science a Natural Science 0:187-194.
Muasya, A. M., J. J. Bruhl, D. A. Simpson, A. Culham, and M. W. Chase. 2000. Suprageneric phylogeny of Cyperaceae: a combined analysis. Pages xiv, 738 in K. L. Wilson and D. A. Morrison, editors. Monocots : systematics and evolution. CSIRO, Collingwood, VIC, Australia.
Muasya, A. M., D. A. Simpson, M. W. Chase, and A. Culham. 1998. An assessment of suprageneric phylogeny in Cyperaceae using rbcL DNA sequences. Plant Systematics & Evolution 211:257-271.
Plunkett, G. M., D. E. Soltis, P. S. Soltis, and R. E. Brooks. 1995. Phylogenetic relationships between Juncaceae and Cyperaceae: Insights from rbcL sequence data. American Journal of Botany 82:520-525.
Reznicek, A. A. 1990. Evolution in Sedges (Carex, Cyperaceae). Canadian Journal of Botany 68:1409-1432.
Reznicek, A. A. 2001. Sectional names in Carex (Cyperaceae) for the flora of North America. Novon 11:454-459.
Roalson, E. H., J. T. Columbus, and E. A. Friar. 2001. Phylogenetic relationships in Cariceae (Cyperaceae) based on ITS (nrDNA) and trnT-L-F (cpDNA) region sequences: Assessment of subgeneric and sectional relationships in Carex with emphasis on section Acrocystis. Systematic Botany 26:318-341.
Starr, J. R., R. J. Bayer, and B. A. Ford. 1999. The phylogenetic position of Carex section Phyllostachys and its implications for phylogeny and subgeneric circumscription in Carex (Cyperaceae). American Journal of Botany 86:563-577.
Waterway, M. J., E. McIntire, and R. G. Olmstead. 1997. Molecular evidence suggests that Carex section Limosae is not monophyletic. American Journal of Botany 84:243.
Yen, A. C., and R. G. Olmstead. 2000. Molecular systematics of Cyperaceae tribe Cariceae based on two chloroplast DNA regions: ndhF and trnL intron-intergenic spacer. Systematic Botany 25:479-494.